Steven F Dowdy
Nature Biotechnology 35, 222–229 (2017) doi:10.1038/nbt.3802
Abstract:
RNA-based therapeutics, such as small-interfering (siRNAs), microRNAs (miRNAs), antisense oligonucleotides (ASOs), aptamers, synthetic mRNAs and CRISPR–Cas9, have great potential to target a large part of the currently undruggable genes and gene products and to generate entirely new therapeutic paradigms in disease, ranging from cancer to pandemic influenza to Alzheimer’s disease. However, for these RNA modalities to reach their full potential, they first need to overcome a billion years of evolutionary defenses that have kept RNAs on the outside of cells from invading the inside of cells. Overcoming the lipid bilayer to deliver RNA into cells has remained the major problem to solve for widespread development of RNA therapeutics, but recent chemistry advances have begun to penetrate this evolutionary armor.
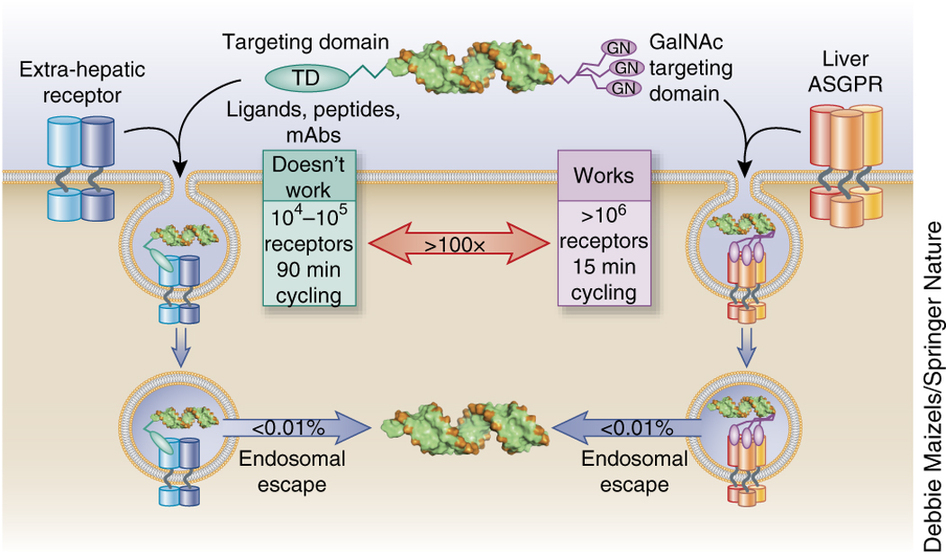
▲ The numerology of endosomal escape.
Tris-GalNAc binding to liver ASGPR (~106/hepatocyte) induces endocytosis (~15 min) where a small fraction of the siRNA or ASO cargo escapes into the cytoplasm to induce selective RNA drug responses. In contrast, targeting non-hepatic cell surface receptors (104–105) that have a much slower rate of endocytosis (~90 min) has proven extremely difficult. Assuming there is no endosomal escape advantage in ASGPR endosomes, ASGPR brings in ~100-fold more siRNAs/ASOs into hepatocytes than is mathematically possible in any other ligand–receptor pair. Consequently, development of next-generation RNA-based therapeutics needs to incorporate new chemistries, materials and/or mechanisms of enhancing endosomal escape ~100-fold.
全文链接:http://www.nature.com/nbt/journal/v35/n3/abs/nbt.3802.html